Overview
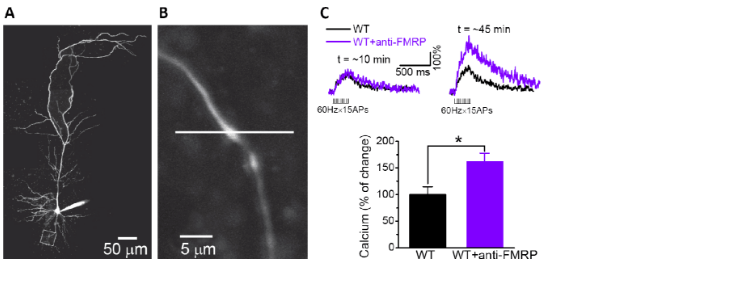
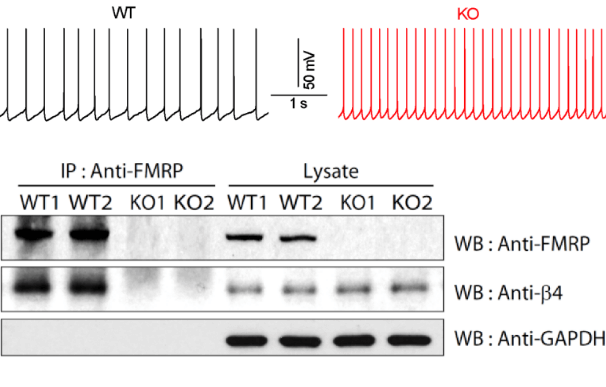
Fragile X syndrome is the most common heritable cause of intellectual disability and autism stemming from the loss of FMRP protein. Two decades of research have focused on the postsynaptic role of FMRP as a regulator of local translation in dendrites. However, clinical trials of therapies based on this mechanism have failed to produce meaningful treatments, and Fragile X syndrome remains incurable. In response to these challenges, we consider the possibility that FMRP has a role in presynaptic processes. Indeed, we have recently identified major defects in neurotransmitter release in a mouse model of Fragile X syndrome.

This discovery revealed the possibility that the FMRP protein may regulate synaptic transmission via direct actions on a variety of ion channels (BK, SK, INaP (persistent-sodium current)), and that the loss of such functions may contribute to the disease phenotypes. Supporting this idea, we were able to link presynaptic FMRP function to specific clinical Fragile X phenotypes (Intellectual disability and seizures) following the discovery of a rare FMRP point mutation identified in a patient with partial Fragile X syndrome. In extending our studies to the circuit level, we found reduced spiking precision and elevated spiking probability caused by a marked excitation/inhibition imbalance. Taken together, our studies have significantly shifted the research landscape surrounding FXS to include consideration of presynaptic pathophysiology.
Progress
Our main objectives going forward are to define the synaptic mechanisms through which synaptic transmission and neural excitability are disrupted by FMRP loss and to define the roles of these presynaptic defects in the pathophysiology of FXS. We will also use electrophysiology to understand excitation/inhibition imbalance in hippocampal and cortical circuits in Fragile X mouse model and to develop strategies to normalize circuit functions. We use a multi-level approach, spanning from single-channel recordings and single-vesicle glutamate release measurements, to cellular and circuit excitability analyses, and behavioral assays. We are also working in collaboration with several labs (Stephen Warren at Emory University, Valeria Cavalli at Washington University) to develop and analyze novel genetic models and rescue strategies for this disorder. We are also working together with Animal Behavior Core at Washington University to translate our findings at the cellular and circuit levels to design and test rescue strategies in behaving animals.